BPP’s drug development initiatives are centered on the invention and novel research findings of Dr. Kim Heidenreich.
In the Heidenreich lab, BPP was the first in CNS research to identify and validate (overturning long held industry beliefs) that leukotrienes, well known as potent pro-inflammatory mediators of the body’s innate immune response to asthma, are synthesized in the brain in the absence of infiltrating immune cells from the periphery.
BPP’s studies utilized a novel injury model developed specifically to mimic mild injury in humans. This model was fully validated to not cause edema, cell damage that could be defined by MRI, or disruption of the blood brain barrier, the “BBB”, (which disruption would have allowed immune cells from the body to enter the brain).
Leukotriene biosynthesis occurs within minutes of brain injury or insult, via a transcellular mechanism wholly dependent on microglia and astrocytes (the brain’s resident immune cells), and neurons (nerve cells). The result of this immune response is the activation and subsequent migration of microglia to the site of injury in the brain, where these activated microglia assess cell damage and express additional pro-inflammatory cytokines and chemokines to foster normal healing processes.
The process by which leukotrienes are synthesized, either in the brain or the body, is defined as the leukotriene pathway. The product of leukotriene biosynthesis is microglial activation and the initiation of neuroinflammation.
BPP studies demonstrated that therapeutic inhibition of the leukotriene pathway using BPP-1001:
- Blocks the synthesis of all four (4) pro-inflammatory leukotrienes in the brain
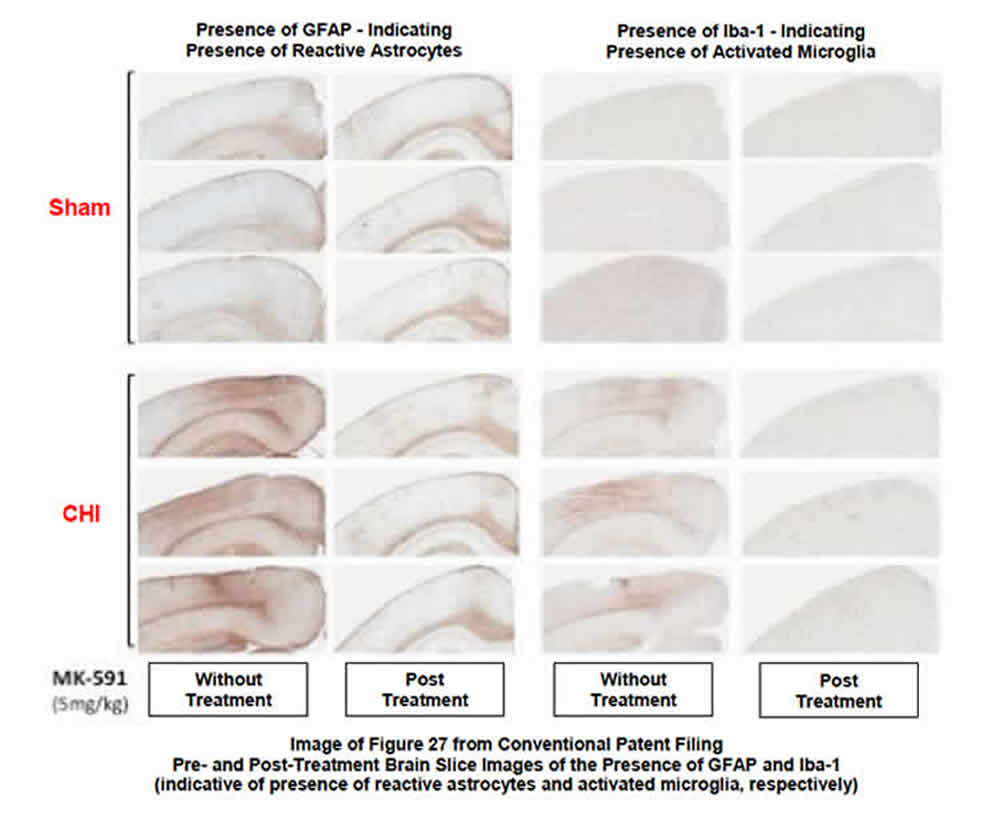
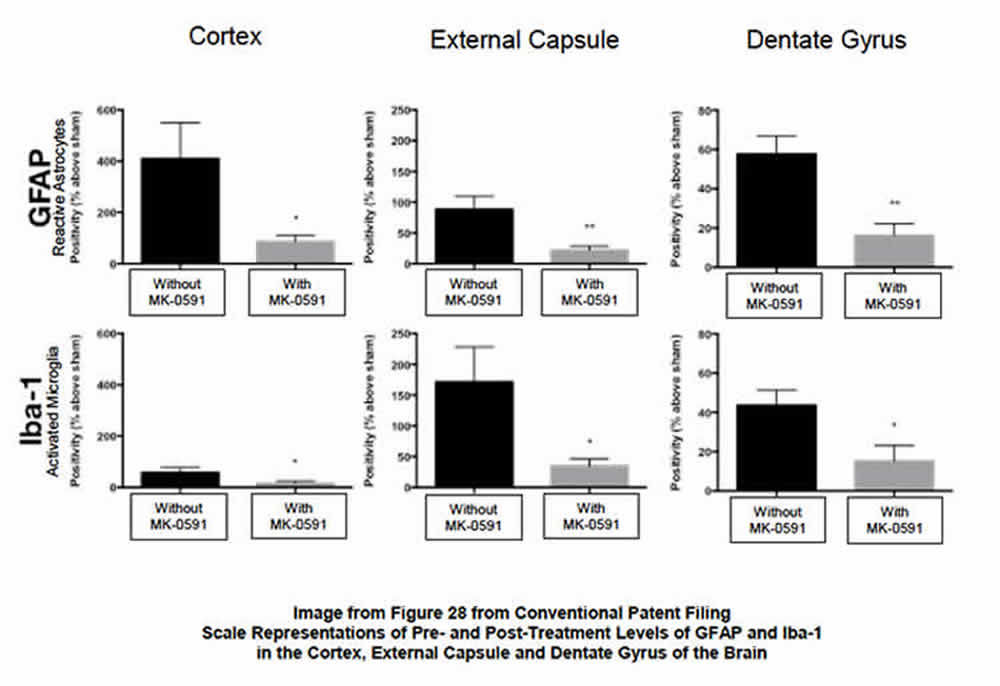
- Significantly reduces levels of activated microglia and reactive astrocytes (elevated levels of which are indicative of the neuroinflammation associated with the brain’s innate immune response). See Images 27 and 28.
- Reduces neuronal cell damage and death, astrocyte cell death, and cognitive deficits, beneficial results which are disease-modifying in most, if not all, neurodegenerative diseases.
Intellectual Property Matters. The groundbreaking results of Kim’s research have been elemental in advancing both BPP’s patent filings and its successful prosecution of key “therapeutic inhibition” claims with the USPTO, as well as the award of US Patent 10,080,748, in September 2018. Intellectual property protection in the US extends into 2035. National Phase Filing prosecutions are underway or pending in the EPO, Canada, Australia, China and India.
Key Terms
Neuroinflammation is the innate inflammatory response within the brain in response to a variety of stimuli, including, among others, bacterial or viral infections, traumatic or acquired brain injury (including stroke), neurodegenerative diseases, autoimmune responses, poisons and other toxins (including the effects of alcohol and drugs), as well as normal aging.
Neuroinflammation is further characterized by acute and chronic processes:
- Acute neuroinflammation is typically short-lived and unlikely to be detrimental to long-term survival of nerve cells.
- Chronic inflammation, by contrast, is a lengthy and often self-perpetuating neuroinflammatory response, particularly in an aging population, that persists long after the initial injury, and may never resolve.
Chronic neuroinflammation includes not only long-standing activation of microglia but subsequent sustained release of inflammatory mediators to perpetuate the inflammatory cycle, activating additional microglia and promoting their proliferation, resulting in further release of inflammatory factors that may cause astrocytes to become “reactive”, which may significantly compromise neuronal survival. These escalating neuroinflammatory cascades are elemental in the pathophysiology of neurodegenerative diseases.
Neurodegeneration is the progressive loss of function and death of nerve cells in the brain (neurons).
Neurodegenerative disease is an umbrella term for a range of conditions that are characterized by the progressive degeneration and death of neurons. These degenerative processes affect balance, movement (ataxias) and mental function, including memory and language (dementias).
Many neurodegenerative diseases, including ALS, MS, Parkinson’s Disease, Alzheimer’s disease and prion disease, occur as a direct result of these degenerative processes. Of these progressive diseases, dementias represent the greatest social, medical and financial burden worldwide. Alzheimer’s Disease comprises approximately 60 to 70 percent of all dementia cases.
Selected Publications
(click link to open)
BPP - 2.11 Neuroinflammation - Publication 2017 - Role of Neuroinflammation in Neurodegeneration
BPP - 2.10 Neuroinflammation - Publication 2016 - Neuroinflammation Pathways - a general review
BPP - 2.8NB Neuroinflammation - Publication 2014 - Neuroinflammation Role and Consequences
BPP - 5.1 Leukotrienes - BPP - Document - The Pharmacology of Leukotrienes
BPP - 5.3 Leukotrienes - BPP - Publication 2007 - Biosynthesis and Metabolism of Leukotrienes
BPP - 5.4 Leukotrienes - BPP - Publication 2007 - Transcellular Synthesis of Leukotrienes
BPP - 5.5 Leukotrienes - BPP - Publication 2009 - Injury Produced CystLTs contribute to Brain Damage
BPP - 5.32 Leukotriene Pathway - Publication 2018 - Leukotriene Pathway - a druggable target in AD
BPP - 7.10 Astrocytes - Publication 2018 - Normal Aging induces A1 pro-inflammatory actiivity
Key Images from the BPP Conventional Patent Filing
BPP’s studies of BPP-1001 in the CNS were focused on the drug’s ability to 1) inhibit the post-injury biosynthesis of leukotrienes, and 2) the resultant reduction of the post-injury numbers and distribution of activated microglia and reactive astrocytes (glial cells that modulate the innate immune response in the brain). The following images provide clarity on the post-therapeutic effect of the drug:
- Image 27 (see below) defines the beneficial effect of BPP-1001 in reducing the post-injury density of both glial cells in the brain. Brain slices harvested from both sham and injured animals as a part of the BPP study identify the significant reduction in both activated microglia and reactive astrocytes after therapy with BPP-1001. The slides also clearly show the remarkable similarity between uninjured and injured-but-treated animals 30 days after injury and treatment.
- Image 28 (see below) quantifies by measurement the post-therapeutic reduction of activated microglia and reactive astrocytes (surrogates for neuroinflammation) in several key areas of the brain.
Summary. The ability of BPP-1001 to substantively reduce the presence of activated microglia and reactive astrocytes in the treated brain after injury, as shown in the two slides, is significant. This result, in addition to the beneficial results found in reducing post-injury neuronal damage and death and cognitive deficits in the study animals, were compelling reasons for the USPTO to award a BPP a patent for the treatment of neuroinflammation and neuroinflammation-mediated damage in the CNS.