BP’s drug development initiatives are centered on the invention and novel research findings of Dr. Kim A. Heidenreich in collaboration with Dr. Robert Murphy, a renowned lipid biochemist.
Kim A. Heidenreich Made a Breakthrough Discovery That Leukotrienes Activate Neuroinflammation
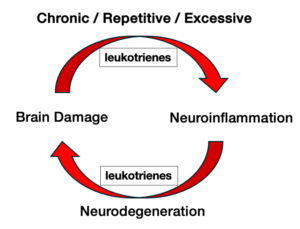
Leukotrienes are undetectable in healthy brain but are detected within minutes following traumatic brain injury. Leukotrienes are produced by the
interaction of ‘activated’ microglia and ‘reactive’ astrocytes in response to brain injury. BPP-1001 blocks leukotriene production and the activation of microglia and astrocytes, thereby preventing:
- brain edema
- neuronal death
- blood-brain-barrier disruption
- synaptic plasticity impairment
- learning and memory deficiencies
BP’s Breakthrough Discovery Has Implications for the Understanding and Treatment of Additional Brain Disorders:
- Traumatic head injury
- Alzheimer’s disease
- Parkinson’s disease
- Multiple sclerosis
- COVID
- Viral encephalitis
- Aging
BP's Solution Interrupts the Uncontrolled Neuroinflammatory Cycle and Allows the Brain to Repair and Recover.
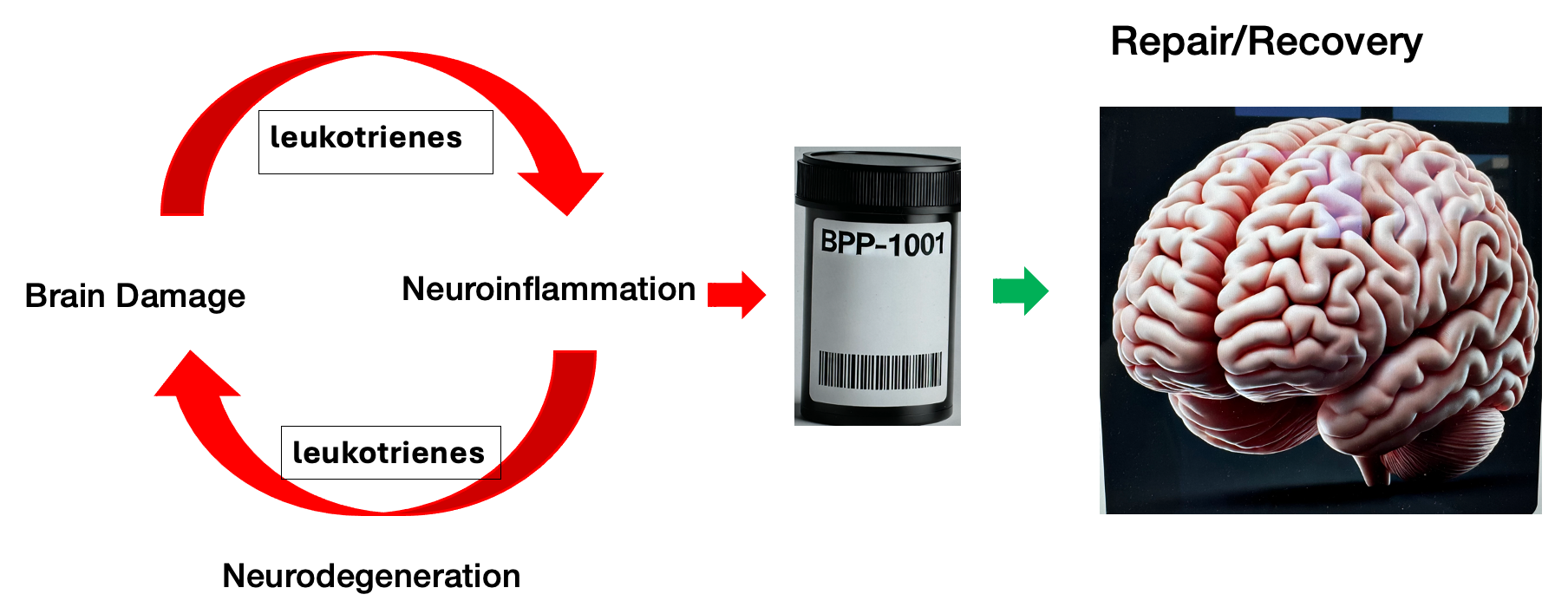
BBP-1001 Has Multiple Attractive Characteristics
- Known mechanism of action
- An anti-inflammatory small molecule that crosses the intact blood-brain-barrier
- Proven safe and effective in humans
- Comprehensive domestic and international patent protection
- Global target markets are large and growing
- Potentially available for FDA fast tracking
- Advantages over monoclonal drugs that are currently being developed for brain injury
Intellectual Property Matters
The patent description is comprehensive and covers “The use of FLAP (5-lipoxygenase activating protein) inhibitors to reduce neurological-inflammation mediated injury in the central nervous system". US Patent 10,080,748. International Patent information available upon request.
Selected Documentation
- BP 5.1 - (Document) Murphy, R. C., Heidenreich KA, et al. - The Pharmacology of Leukotrienes
- BP 5.3 - (Publication 2007) Robert C. MURPHY and Miguel A. GIJON - Biosynthesis and metabolism of leukotrienes
- BP 5.4 - (Publication 2007) Santiago E. Farias, Simona Zarini, Thomas Precht, Robert C. Murphy and Kim A. Heidenreich - Transcellular biosynthesis of cysteinyl leukotrienes in rat neuronal and glial cells
- BP 5.5 - (Publication 2009) Santiago Farias, Lauren C. Frey, Robert C. Murphy, and Kim A. Heidenreich - Injury-Related Production of Cysteinyl Leukotrienes Contributes to Brain Damage following Experimental Traumatic Brain Injury
- BP 5.6 - (Publication 2014) Corser-Jensen, C.E., D.J. Goodell, P. Šerbedžija, R.K. Freund, and K.A. Heidenreich - Inhibition of leukotriene production protects against secondary brain damage and cognitive deficits induced by traumatic brain injury
- BP 5.7 - (Chapter 2017) Heidenreich, KA and Corser-Jenson, CE - 5-Lipoxygenase activating protein (FLAP) inhibitors are promising therapeutics for acute and chronic neuroinflammation following traumatic brain injury IN New Therapies for Traumatic Brain Injury: Prevention of Secondary Brain Damage and Enhancement of Repair and Regeneration
Key Images from the BP Conventional Patent Filing
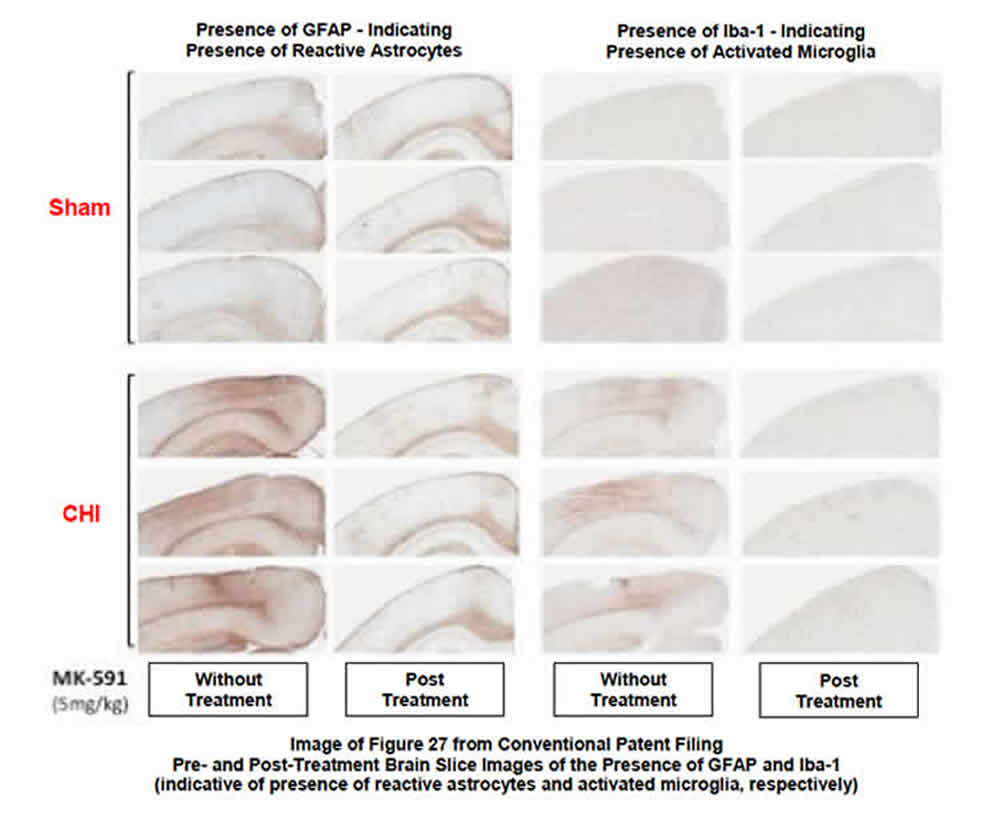
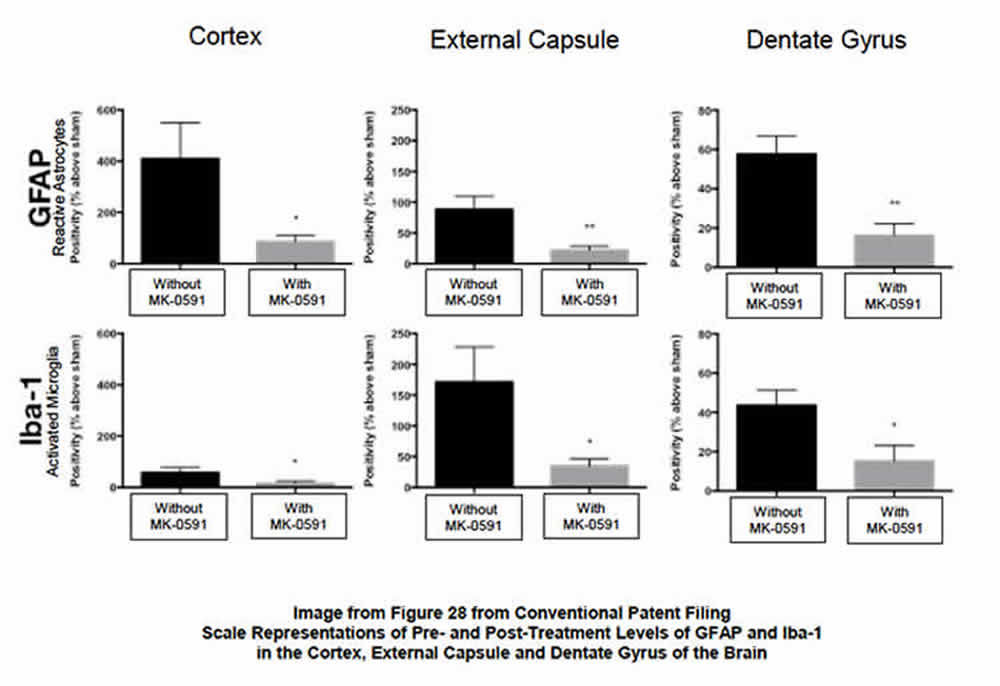
BP’s studies of MK-591--repurposed as BPP-1001--in the CNS were focused on the drug’s ability to 1) inhibit the post-injury biosynthesis of leukotrienes, and 2) the resultant reduction of the post-injury numbers and distribution of activated microglia and reactive astrocytes (glial cells that modulate the innate immune response in the brain). The following images provide clarity on the post-therapeutic effect of the drug:
- Top image (see below) shows representative images of reactive astrocytes and activated microglia in brains from injured animals, with and without treatment by BPP-1001. Brain slices harvested from both sham (control) and injured animals as a part of the BPP study identify the significant reduction in both activated microglia and reactive astrocytes after therapy with BPP-1001. The slides also clearly show the remarkable similarity between uninjured and injured-but-treated animals 30 days after injury and treatment.
- Bottom image (see below) quantifies the post-therapeutic reduction of activated microglia and reactive astrocytes (surrogates for neuroinflammation) in several key areas of the brain.
Summary. The ability of BPP-1001 to substantively reduce the presence of activated microglia and reactive astrocytes in the treated brain after injury is significant.